

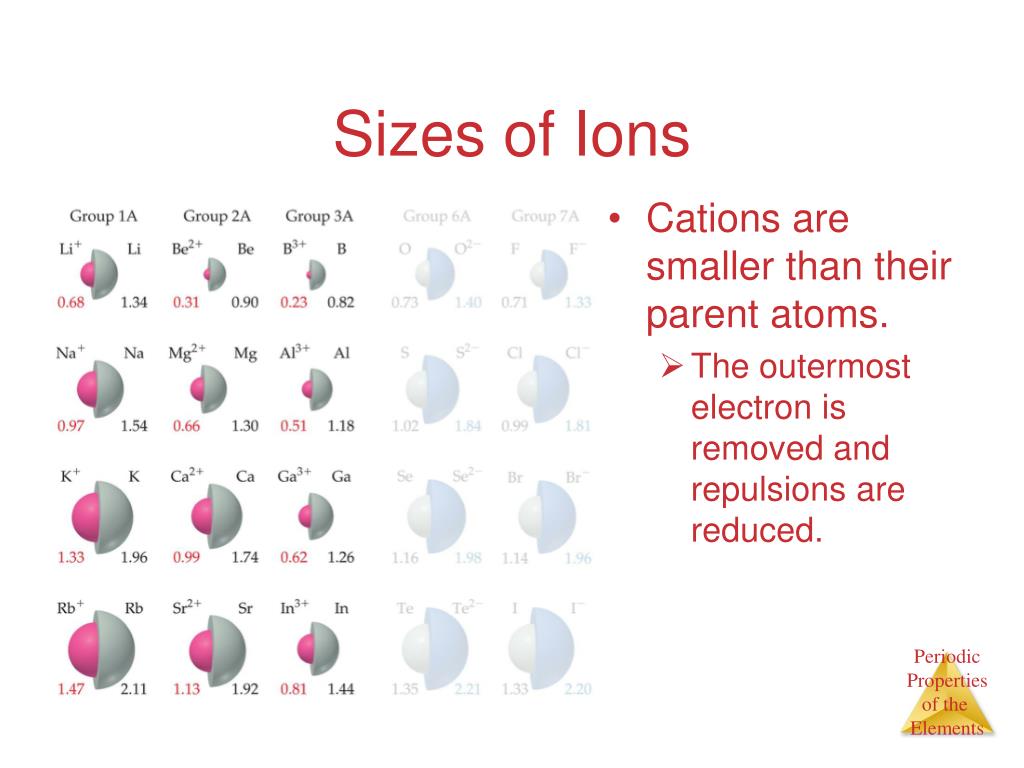
Distance from center to the outer most region in ion.Each new period begins with new energy level which is farther from nucleus, increasing atomic radius. Atomic radius increase down group from top to bottom.
Nucleus’s positive charge is greater relative to charge of electrons in same shell, pulling electrons closer to nucleus and thus reducing atomic radius Atomic radius decreases across period from left to right.Way of finding radius would be to measure distance between two atoms in diatomic molecule X 2 and divide by two for the radius.Bohr model has orbits, thus radius can be measured easily however we know that this is highly simplistic.Distance from center to the outer most region.Atomic properties show pattern amongst the period table which can be classified as trends 6 trends need to know: D.7 (HL) Taxol – a chiral auxiliary case studyĮlectron configuration help determine atomic properties.D.6 Environmental impact of some medications.D.1 Pharmaceutical products and drug action.C.8 Photovoltaic and dye-sensitized solar cells.C.6 Electrochemistry, rechargeable batteries and fuel cells.C.5 Environmental impact – global warming.A.10 Environmental impact – heavy metals.A.8 Superconducting metals and X-ray crystallography.
A.2 Metals and inductively coupled plasma (ICP) spectroscopy.21.1 Spectroscopic identification of organic compounds.18.2 Calculations involving acids and bases.16.1 Rate expression and reaction mechanism.14.1 Covalent bonding and electron domain and molecular geometrics.The periodic table – the transition metals 11.3 Spectroscopic identification of organic compounds.11.1 Uncertainties and errors in measurements and results.6.1 Collision theory and rates of reaction.1.1 Introduction to the particulate nature of matter and chemical change.


 0 kommentar(er)
0 kommentar(er)
